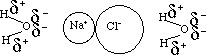
Chapter 19: Chemical Interactions
Four types of chemical interactions: (strongest to weakest)
ion - dipole
dipole - dipole
dipole - induced dipole
induced dipole - induced dipole
These are all much weaker (over
100 times) than covalent or ionic bonds.
Ion - dipole
In many molecules, electrons are distributed closer to one side than the other. This causes one side to be slightly more negative than the other. We call this type of molecule a dipole.
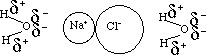
ionic - dipole interaction: sodium
chloride is an ionic compound
Dipole - dipole
When dipoles interact between themselves we have a dipole - dipole interaction. The hydrogen bond relatively strong.

Dipole - induced dipole
When a molecule with no dipole
(such as O2) is
placed near a molecule that has a dipole (H2O) it may create an induced dipole in a molecule that
has no dipole. The induced dipole is temporary, existing only as long
as the dipole is close to the other molecule.
Induced dipole - induced dipole
The random motion of the electrons
surrounding a nonpolar element or molecule makes it possible for more
electrons to gather on one side than the other. This creates a
temporary dipole, which can induce a dipole in other nearby nonpolar
molecules which can in turn induce yet more dipoles ...
The different types of
interactions help explain some of the physical properties of a
substance. A solid is formed when the chemical interactions between
atoms or molecules is strong enough to hold them in a fixed
arrangement. As the interactions between molecules are become
unfixed, molecules keep shifting around to interact with other
molecules, the compound becomes a liquid. As more shifting takes
place, the compound becomes a gas.
Special properties of water
As a solid (ice) its molecules
form a crystal, taking up more volume than an equivalent mass of
liquid water just above the freezing temperature. Water has a high
heat capacity due to the strength of the hydrogen bond.
In a liquid, molecules at the
surface are attracted to one another. A force must be applied in
order for a object to penetrate the surface. The energy need to do
this is referred to as the surface tension of the liquid. Surface
tension also explains why liquids form spherical drops and why a
liquid spilled onto a flat surface will hold together.
Adhesive force: chemical interactions that arise between two different substances.
Cohesive force: chemical
interactions that arise between like substances.
Solution: a homogeneous mixture consisting of only one phase. i.e. sugar water and air.
Solvent: the component of the solution with the greatest quantity.
Solute: all the other components in the solution.
Example: mix 50 grams of sugar in
100 grams of water. Solvent is water and solute is sugar.
The quantity of solute dissolved in a solution is often referred to by its concentration:
concentration = (amount of solute)/(volume of solution)
Example: mix 50 grams of sugar in
100 grams of water and you get a concentration of 0.5 grams of sugar
per liter.
Chemists are usually more
interested in the number of molecules of solute rather than the
number of grams. The unit of use is the mole.
Solubility: ability of a solute to dissolve in a solvent. Temperature affects solubility. In general, the higher the temperature of the solvent, the easier it is to dissolve the solute. Also with higher temperatures, more of the solute can be dissolved.